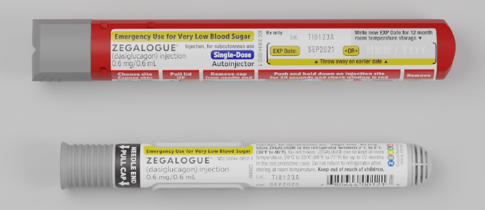
Zegalogue (also known as dasiglucagon injection) was recently approved by the FDA to treat severe hypoglycemia(low BG) in people with diabetes over the age of six. Zegalogue is made by Zealand Pharma, a biotechnology company founded in Denmark in 1998. The new emergency glucagon will be available starting in June as a pre-filled syringe or an auto-injector device.
Clinical trials conducted, found that Zegalogue would raise glucose levels and resolve extreme hypoglycemia much faster than traditional glucagon. The data shows that taking Zegalogue (0.6 mg) during an extreme case of hypoglycemia would raise blood glucose levels by at least 20 mg/dl within an average of ten minutes. In comparison, traditional glucagon injections can take 30-45 minutes to achieve this same effect.
Almost all adults in the trial (99% of participants) recovered and returned to non-hypoglycemic blood glucose levels within 15 minutes of taking Zegalogue.
Zegalogue along with Baqsimi and Gvoke are a next-generation rescue glucagon. Each of these three glucagon are labeled “next generation due to them working without the need to mix dry powder and liquid.