65-OR: Remote Monitoring of CGM Data at Diabetes Camp Mitigates Hypoglycemia Day and Night
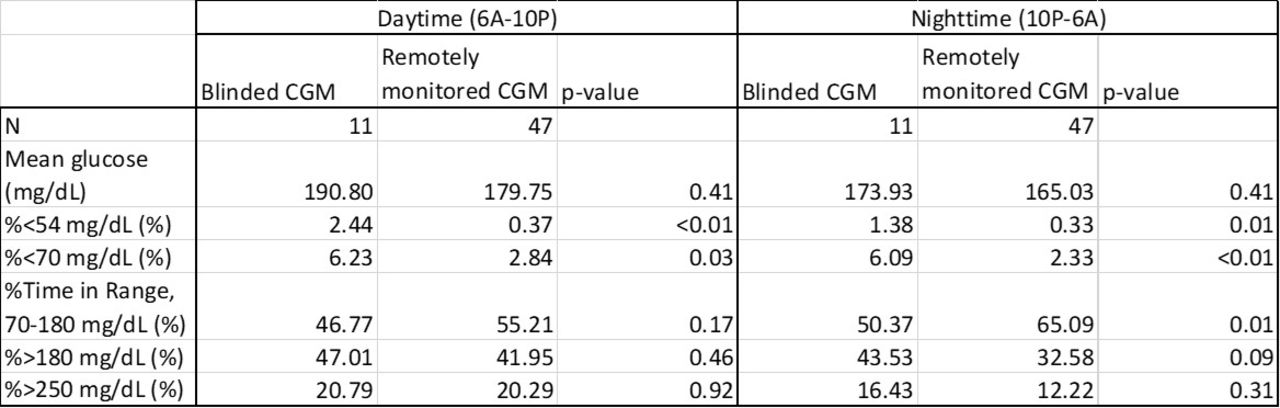
Some diabetes camps require campers to relinquish diabetes technology upon arrival. We evaluated the acceptability of continuous glucose monitoring (CGM) use and remote monitoring of CGM data at camp. Insulin-treated campers ages 7-18 years were enrolled at a week-long diabetes camp in the U.S. All campers performed regular fingerstick testing, which informed treatment decisions. Campers that monitored their glucose with fingerstick testing at home were fitted with blinded Dexcom CGM at camp. Campers that used Dexcom CGM Systems with the capacity to share data at home continued to use their self-supplied systems; shared data were displayed on the Follow app on counselor-carried smart devices and on a centralized display in the medical cabin. Remote monitoring informed staff of glucose values 250 mg/dL, prompting fingerstick testing outside of regular intervals. Data were compared between blinded and remotely monitored, non-blinded CGM systems. Remote monitoring of CGM data was associated with significant reductions in hypoglycemia and improvements in overnight time in range (Table). Remote monitoring facilitated 73 interventions overnight that may have otherwise been missed by fingerstick testing. Remote monitoring of CGM data at diabetes camp allows implementation of tighter target glucose ranges, facilitates timely hypoglycemia treatment, and should be encouraged at all diabetes camps.
Disclosure S.E. Gleich: Research Support; Self; Dexcom, Inc. N.D. Gibson-North: None. S. Puhr: Employee; Self; Dexcom, Inc. J. Welsh: Employee; Self; CSL Behring. T.C. Walker:Employee; Self; Dexcom, Inc. D. Caruso: None.
- © 2020 by the American Diabetes Association
http://www.diabetesjournals.org/content/license
Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered. More information is available at http://www.diabetesjournals.org/content/license.